An Indo-German Biotech Company, Vedicinals India Pvt Ltd (VIPL) from Pune, Maharashtra has made available
VEDICINALS®-9 for online purchase through their own website. This is a Nutraceutical suspension, comprising 9 highly purified, standardized, bio active molecules. All 9 molecules are added to maximum possible concentration with a very high safety profile of the formulation with LD 50 Value = Class 5, >5000 mg/kg.
VEDICINALS®-9 was evaluated during a CTRI, WHO registered clinical trial and proved statistically significant & clinically effective as an adjuvant to SOC treatment of Covid-19 positive patients. Additionally, Post/Long Covid prevention was evaluated and showed good results.
VIPL has uploaded a lot of valuable information, research data and papers relevant to Covid-19/ conditions arising from the infection on their website
https://www.vedicinals.com/ Researchers & Universities are joining in to publish papers on the formulation/ ingredients together with VIPL. VIPL has documented 71 drug target pathways relevant to Covid-19 and still have 12 more to upload. It’s a treasure thrown open to global scientific community to fight the pandemic unitedly.
The Chairman of VIPL Joachim Gerlach conveyed to Industry Outlook:
"Late January 2020, observing what happened in China, when we got our first credible inputs (Genetic structure of Novel Corona Virus published by China) we assembled the most capable teams of experts we had in our global networks. “All hands-on deck!!” so to speak.

When we studied thoroughly the genetic structure of new corona virus, and compared it with old strains like SARS, MERS, we realized we are up against a very smart capable enemy which is in stealth mode, having survived since 2004 and is mutating/adapting ever since. We started with assumption/possibility that this virus is going to mutate endlessly and be with us for a very long time.
Our teams focused on mapping all the mutations documented/available so far and came up with a report on most/least mutated sites on virus and estimations based on shown trends to mutate.
Also, we were learning new things about the capabilities of the virus and the ways in which it would harm humans. Based on all the global inputs and our team’s estimations/proposal we finalized our target of formulation to have minimum three capabilities as the following:
#1 Anti-Viral
#2 Anti-inflammatory
#3 Organ protective
Fortunately, we were working on Therapeutics for the treatment of inflammatory issues, organ damage recovery caused by environmental pollution and almost ready to launch the product MOLECUSAN capable of handling #2 & #3. This gave us pole position as we had to only work on selecting the best Anti-viral molecules to be added to our mix. The global scientific community was working hard, with lot of efforts targeting Spike Protein.
Looking at the evasive tactics of the virus, possible mutations and the spike protein being
already targeted by established industry leaders, we decided to work on another virus protease.
We confirmed 3cLPro/MPro is very promising drug target site on Virus being very little to no mutations and we choose this as our Anti Viral drug target. By end of April 2020, we were ready with our formulation design. Since April 2020 we are continually monitoring the mutations happening and we are lucky so far, even including Delta we are still hitting right on target.
Our choice of 3cL Pro/ M-Pro was out of box and proving beneficial. Apart from 3cL Pro/ M-Pro we have covered other 71 documented drug target pathways addressed by VEDICINALS®-9 and hope to serve humanity.
The combination of German development skills and Indian execution capabilities made this a unique project concluding within a record timeframe.
Designed in Europe, Developed & Made in India
Most rewarding for our teams is the outcome of the trials and the quality of currently incoming data / feedback. The clinical trial involved 124 patients. Almost half used the nutraceutical product VEDICINALS®-9 alongside standard approved Covid-19 treatment. These patients showed better clinical outcomes including faster viral clearance, accelerated repair of lung damage, prevention of severe Covid-19 disease progression, improvements of various key biomarkers such as IL-6 and CRP, faster resolution of clinical symptoms such as fever, cough, fatigue etc and organ protection.
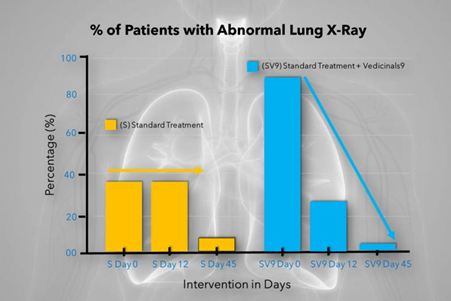
The chart shows the percentage of abnormal lung X-Ray in both groups of human clinical trials.
In the Vedicinals 9 treatment group there was a rapid and significant improvement on the documented X-Ray findings. This correlates with the fast Viral clearance, Anti-inflammatory and Organ protective data we got parallel from other biomarker evaluations.
Arrest of disease progression, increase of oxygen levels and regeneration of organ tissues are documented. For further information, please log on to our website.
VIPL filed a patent application early last year, along with PCT. The trademark is registered in India and applied for registration in many countries of interest. Many Indian companies have contributed to converting the concept into a real life, shelf ready product with all necessary compliance. VIPL expresses its gratitude to all the partner companies and associates of this project.
VEDICINALS®-9, Suspension with 9 different molecules, high individual dosages, formulation development done at Tirupati Group in record time, is unsurpassed in its bioavailability and formulation stability. It is being sold at a net price of US$ 135 for the Indian market for a set of 14 bottles, covering a complete 14-day treatment. The product is priced much higher in overseas markets.
VIPL is convinced that they will make a significant difference in the further course of this pandemic and are welcoming other companies, regulatory agencies to join the forces and seek combination treatment options with VEDICINALS®-9.
SARS COV2 and its new variants will need to be controlled by combining vaccines, pharmaceutical drugs, antibody treatment, oxygen supply, all in different combinations, like HIV treatment.
"We have already entered the battle.”
Prakash Salunke, Managing Director and Shareholder said, VEDICINALS®-9 is certified as a Nutraceutical product in India by the FSSAI, also we have applied for upgrade to FSMP.
Our next obvious step is to continue further studies and trials to meet the requirements for compliance and certification of VEDICINALS®-9 as a Phyto-pharmaceutical product as it was designed from ground up as Phyto-pharmaceutical in the first place.
We are in contact with government agencies and getting proper guidance and encouragement a startup can imagine. We are already receiving overwhelming response for VEDICINALS®-9 due to its efficacy and are now in the process of preparing to scale up production along with our partners.
We are counting on the quality of this product and the networking capabilities of the Indian people to share on their experiences and will be proud to help so many families.”

